Q fever
| Q fever | |
|---|---|
| Classification and external resources | |
| ICD-10 | A78. |
| ICD-9 | 083.0 |
| eMedicine | med/1982 ped/1973 |
| MeSH | D011778 |
Q fever is a disease caused by infection with Coxiella burnetii,[1] a bacterium that affects humans and other animals. This organism is uncommon but may be found in cattle, sheep, goats and other domestic mammals, including cats and dogs. The infection results from inhalation of contaminated particles in the air, and from contact with the milk, urine, feces, vaginal mucus, or semen of infected animals. Rarely, the disease is tick borne. [2] The incubation period is 9–40 days. It can be considered the most infectious disease in the world, as a human being can be infected by a single bacterium.[3] The bacterium is an obligate intracellular pathogen.
Contents |
History
It was first described by Edward Holbrook Derrick[4] in abattoir workers in Brisbane, Queensland, Australia. The "Q" stands for "query" and was applied at a time when the causative agent was unknown; it was chosen over suggestions of "abattoir fever" and "Queensland rickettsial fever", to avoid directing negative connotations at either the cattle industry or the state of Queensland.[5]
The pathogen of Q fever was discovered in 1937, when Frank Macfarlane Burnet and Mavis Freeman isolated the bacterium from one of Derrick’s patients.[6] It was originally identified as a species of Rickettsia. H.R. Cox and Davis isolated it from ticks in Montana, USA in 1938.[7] It is a zoonotic disease whose most common animal reservoirs are cattle, sheep and goats. Coxiella burnetii is no longer regarded as closely related to Rickettsiae but as similar to Legionella and Francisella and is a proteobacterium.
Manifestations
Incubation period is usually 2 to 3 weeks. The most common manifestation is flu-like symptoms with abrupt onset of fever, malaise, profuse perspiration, severe headache, myalgia (muscle pain), joint pain, loss of appetite, upper respiratory problems, dry cough, pleuritic pain, chills, confusion and gastro-intestinal symptoms such as nausea, vomiting and diarrhea. The fever lasts approximately 7 to 14 days.
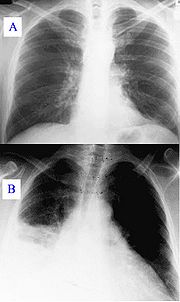
During the course, the disease can progress to an atypical pneumonia, which can result in a life threatening acute respiratory distress syndrome (ARDS), whereby such symptoms usually occur during the first 4 to 5 days of infection.
Less often the Q fever causes (granulomatous) hepatitis which may be asymptomatic or becomes symptomatic with malaise, fever, liver enlargement (hepatomegaly) and pain in the right upper quadrant of the abdomen. Whereas transaminase values are often elevated, jaundice is uncommon. Retinal vasculitis is a rare manifestation of Q fever.[8]
The chronic form of Q fever is virtually identical to inflammation of the inner lining of the heart (endocarditis),[9] which can occur months or decades following the infection. It is usually fatal if untreated. However, with appropriate treatment the mortality falls to around 10%.
Appearance and incidence
The pathogenic agent is to be found everywhere except New Zealand.[10] In Europe it appears as hepatitis rather than pneumonia as in the United States. The bacterium is extremely sustainable and virulent: a single organism is able to cause an infection. The common way of infection is inhalation of contaminated dust, contact with contaminated milk, meat, wool and particularly birthing products. Ticks can transfer the pathogenic agent to other animals. Transfer between humans seems extremely rare and has so far been described in very few cases.
Some studies have shown more men to be affected than women,[11][12] which may be attributed to different employment rates in typical professions.
"At risk" occupations include, but are not limited to:
- veterinary personnel
- stockyard workers
- farmers
- shearers
- animal transporters
- laboratory workers handling potentially infected veterinary samples or visiting abattoirs
- people who cull and process kangaroos
- hide (tannery) workers.
Diagnosis
Diagnosis is usually based on serology[13][14] (looking for an antibody response) rather than looking for the organism itself. Serology allows to detect chronic infection as high antibody levels are found against the virulent form of the bacterium. Molecular detection of bacterial DNA is increasingly used. Culture is technically difficult and not routinely available in most microbiology laboratories.
Q fever can cause endocarditis (infection of the heart valves) which may require transoesophageal echocardiography to diagnose. Q fever hepatitis manifests as an elevation of ALT and AST, but a definitive diagnosis is only possible on liver biopsy which shows the characteristic fibrin ring granulomas.[15]
Treatment
Treatment of the acute Q fever with antibiotics is very effective and should take place in consultation with an infectious diseases specialist. Commonly used are doxycycline, tetracycline, chloramphenicol, ciprofloxacin, ofloxacin, and hydroxychloroquine. The chronic form is more difficult to treat and can require up to four years of treatment with doxycycline and quinolones or doxycycline with hydroxychloroquine.
Q fever in pregnancy is especially difficult to treat because doxycycline and ciprofloxacin are contraindicated in pregnancy. The preferred treatment is five weeks of co-trimoxazole.[16]
Prevention
Protection is offered by Q-Vax, a whole cell inactivated vaccine developed by an Australian vaccine manufacturing company CSL.[17]. The intradermal vaccination is composed of killed Coxiella burnetii organisms. Skin and blood tests should be done before vaccination to identify preexisting immunity; the reason is that vaccinating subjects who already have an immunity can result in a severe local reaction. After a single dose of vaccine, protective immunity lasts for many years. Revaccination is not generally required. Annual screening is typically recommended.[18]
In 2001, Australia introduced a national Q fever vaccination program for people working in "at risk" occupations.
The Soviet Union had earlier developed a killed vaccine but its side effects prevented its licensing abroad.
Biological warfare
Q fever has been described as a possible biological weapon.[19]
The United States investigated Q fever as a potential biological warfare agent in the 1950s with eventual standardization as agent OU. At Fort Detrick and Dugway Proving Ground human trials were conducted on Whitecoat volunteers to determine the median infective dose (18 MICLD50/person i.h.) and course of infection. As a standardized biological it was manufactured in large quantities at Pine Bluff Arsenal, with 5,098 gallons in the arsenal in bulk at the time of demilitarization in 1970.
Q fever is a category "B" agent.[20] It can be contagious and is very stable in aerosols in a wide range of temperatures. Q fever microorganisms may survive on surfaces up to 60 days.
References
- ↑ Beare PA, Samuel JE, Howe D, Virtaneva K, Porcella SF, Heinzen RA (April 2006). "Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons". J. Bacteriol. 188 (7): 2309–24. doi:10.1128/JB.188.7.2309-2324.2006. PMID 16547017. PMC 1428397. http://jb.asm.org/cgi/pmidlookup?view=long&pmid=16547017.
- ↑ "Q fever". http://www.cdc.gov/ncidod/dvrd/qfever/.
- ↑ Q fever caused by Coxiella burnetii
- ↑ Derrick EH. Q" fever a new fever entity: clinical features. diagnosis, and laboratory investigation. Med J Aust. 1937;11:281-299.
- ↑ Joseph E. McDade (1990). "Historical Aspects of Q Fever". In Thomas J. Marrie. Q Fever, Volume I: The Disease. CRC Press. p. 8. ISBN 0849359848.
- ↑ Burnet FM, Freeman M. Experimental studies on the virus of “Q” fever. Med J Aust 1937; 2: 299-305.
- ↑ Davis, G. E., and H. R. Cox. 1938. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersonii, reactions in animals, and filtration. Public Health Rep. 53:2259-2282.
- ↑ Kuhne F, Morlat P, Riss I, et al. (1992). "Is A29, B12 vasculitis caused by the Q fever agent? (Coxiella burnetii)" (in French). J Fr Ophtalmol 15 (5): 315–21. PMID 1430809.
- ↑ Karakousis PC, Trucksis M, Dumler JS (June 2006). "Chronic Q fever in the United States". J. Clin. Microbiol. 44 (6): 2283–7. doi:10.1128/JCM.02365-05. PMID 16757641. PMC 1489455. http://jcm.asm.org/cgi/pmidlookup?view=long&pmid=16757641.
- ↑ Cutler SJ, Bouzid M, Cutler RR (April 2007). "Q fever". J. Infect. 54 (4): 313–8. doi:10.1016/j.jinf.2006.10.048. PMID 17147957. http://linkinghub.elsevier.com/retrieve/pii/S0163-4453(06)00378-1.
- ↑ Domingo P, Muñoz C, Franquet T, Gurguí M, Sancho F, Vazquez G (October 1999). "Acute Q fever in adult patients: report on 63 sporadic cases in an urban area". Clin. Infect. Dis. 29 (4): 874–9. doi:10.1086/520452. PMID 10589906. http://www.journals.uchicago.edu/doi/abs/10.1086/520452?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dncbi.nlm.nih.gov.
- ↑ Dupuis G, Petite J, Péter O, Vouilloz M (June 1987). "An important outbreak of human Q fever in a Swiss Alpine valley". Int J Epidemiol 16 (2): 282–7. doi:10.1093/ije/16.2.282. PMID 3301708. http://ije.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=3301708.
- ↑ Maurin M, Raoult D (October 1999). "Q fever". Clin. Microbiol. Rev. 12 (4): 518–53. PMID 10515901. PMC 88923. http://cmr.asm.org/cgi/pmidlookup?view=long&pmid=10515901.
- ↑ Scola BL (October 2002). "Current laboratory diagnosis of Q fever". Semin Pediatr Infect Dis 13 (4): 257–62. doi:10.1053/spid.2002.127199. PMID 12491231. http://linkinghub.elsevier.com/retrieve/pii/S104518700250008X.
- ↑ van de Veerdonk FL, Schneeberger PM. (2006). "Patient with fever and diarrea". Clin Infect Dis 42: 1051–2. doi:10.1086/501027.
- ↑ Carcopino X, Raoult D, Bretelle F, Boubli L, Stein A (2007). "Managing Q fever during pregnancy: The benefits of long-term Cctrimoxazole therapy". Clin Infect Dis 45 (5): 548–555. doi:10.1086/520661. PMID 17682987.
- ↑ csl.com.au
- ↑ [1]
- ↑ Madariaga MG, Rezai K, Trenholme GM, Weinstein RA (November 2003). "Q fever: a biological weapon in your backyard". Lancet Infect Dis 3 (11): 709–21. doi:10.1016/S1473-3099(03)00804-1. PMID 14592601. http://linkinghub.elsevier.com/retrieve/pii/S1473309903008041.
- ↑ Seshadri R, Paulsen IT, Eisen JA, et al. (April 2003). "Complete genome sequence of the Q-fever pathogen Coxiella burnetii". Proc. Natl. Acad. Sci. U.S.A. 100 (9): 5455–60. doi:10.1073/pnas.0931379100. PMID 12704232. PMC 154366. http://www.pnas.org/cgi/pmidlookup?view=long&pmid=12704232.
External links
- Q fever at the CDC
- Coxiella burnetii genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
|
||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||